Margan Clinical Research Organization (MMARCRO) is an organization which is based in Accra Ghana and set up to provide meticulous and innovative Phase I-IV clinical trial management and monitoring services to pharmaceutical, biotechnology and medical device companies. The CEO, Margaret Williams started working in clinical trials as a clinical trial study coordinator on the Rotavirus project for the RotateqTM vaccine. Both the CEO and her Associate Dr. Elizabeth Diallo worked as senior clinical trial monitors on the Meningitis Vaccine Project (MVP) for the MenAfriVac vaccine. The Meningitis Vaccine Project is the only infant study which led to the licensing of MenAfriVac vaccine for infants for incorporation into routine EPI programmmes across African countries in the meningitis belt in early 2016. The MenAfriVac vaccine has been used to immunize over 275 million in 16 African countries across the meningitis belt.
As a provider of clinical research monitoring services, we have set up standards of excellence for the provision of remote and on-site monitoring activities and aim to become the go-to Clinical Research Organization for delivery of quality and responsive clinical monitoring services to our clients at all times
As a strategic partner with our clients, our mission is to provide excellence in clinical monitoring services to help our clients excel at achieving optimal outcomes in the clinical study protocols, which they develop to provide investigational devices, vaccines and drugs to improve health outcomes in all societies.
Margaret Williams, CEO Margaret Williams is a Senior Clinical Research Associate and Chief Executive Officer of Margan Clinical Research Organization (MMARCRO). She received her MPH degree from the University of Ghana, Legon (2007), and her CRA graduate certification from the Michener Institute for Applied Health Sciences, Canada (2011) and also has extensive post graduate training in Research Ethics and Good Clinical Practice. She has worked as a Contract Clinical Research Associate for the Swiss Tropical and Public Health Institute, (Swiss TPH) in Basel, Switzerland, Agence Africaine de Recherché en Santé Humaine (AARSH) and Santé Plus in Dakar Senegal and Kendle Services in South Africa. Her experience in phase I to IV clinical trials co-ordination and monitoring since 2007 has involved clinical research in a variety of infectious diseases including rotavirus diseases, meningitis, tuberculosis and malaria. Dr. Elizabeth Liyong Diallo, Associate Dr. Elizabeth Diallo is a Medical Doctor and Senior Clinical Research Associate. She is a Cameroonian based in Dakar, Senegal and is fluent in English and French with a basic understanding of the German language. She is a senior associate of the Margan Clinical Research Organization (MMARCRO) and has worked as a Contract Clinical Research Associate for the Swiss Tropical and Public Health Institute, (Swiss TPH) in Basel, Switzerland, Agence Africaine de Recherché en Santé Humaine (AARSH) and Santé Plus in Dakar Senegal and Clinical Research Africa in Kenya. Her extensive post graduate training includes training in Research Ethics, Good Clinical Practice and Vaccinology. Her experience in monitoring phase II to IV clinical trials since 2007 has involved clinical research in a variety of infectious diseases including meningitis, tuberculosis HIV and malaria Philip Ayivor, Certified Nurse & Contract Clinical Research Associate Phillip Ayivor is a certified nurse and a Contract Clinical Research Associate for Margan Clinical Research Organization (MMARCRO). He received his MPH degree from the University of Ghana, Legon (2007) and has had extensive post graduate training in Research Ethics and Good Clinical Practice. His experience in phase II to IV clinical trials has involved clinical research co-ordination and monitoring in a variety of diseases including HIV, meningitis, and malaria since February 2003. MMARCRO Code of Conduct is built upon the following principles: It is the policy of MMARCRO to conduct its business fairly, relying on the merits of its services and people to maintain the highest standards of ethics and integrity at all levels at all times. All MMARCRO’s recruitment policies and practices are fully legal, ethical and in-line with global best practices. We have a zero tolerance approach for any sort of exploitation of our employees. MMARCRO has developed the under listed policies on compliance to cover the following areas in for ethical business conduct

The Rotavirus study resulted in the licensing of RotateqTM vaccine which is now part of the routine expanded program of immunization (EPI) vaccines in Africa.Teamwork is essential to meet our individual and corporate goals

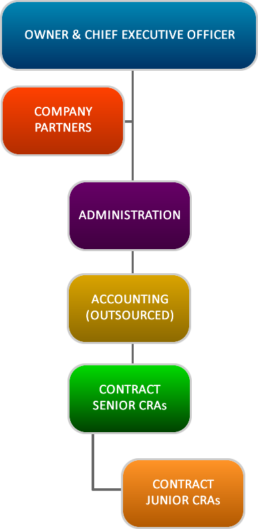
Our Team

Margan Clinical Research Organization
She is a Ghanaian, based in Accra in the Greater Accra Region of Ghana and is fluent in English and Akan. She also has a basic understanding of the French Language
Her vision to provide meticulous clinical trial monitoring in both Anglophone and Francophone countries enabled the strategic partnership between her and her Associate Dr Elizabeth Diallo.

Margan Clinical Research Organization

Margan Clinical Research Organization
He is a Ghanaian, based in Dodowa in the Eastern Region of Ghana and is fluent in English, and a variety of local Ghanaian languages. He also has a basic understanding of the French languageCode of Conduct
We are fully aligned with our clients’ mission, and committed to achieving results in spite of any challenges we may face.
We demonstrate responsibility by weighing the implications and consequences of every action and holding ourselves accountable for the outcome. This motivates us to think quickly, and make conscientious decisions.
We respect the local cultures traditions and mores in the regions where we operate, and we promote a tolerant, harassment-free internal culture where all employees are treated respectfully and equally.
We display integrity in our behavior at all times, from the corporate level to the individual employee. We place strong emphasis on protecting intellectual property and confidential information and adhere to the highest standards of integrity when interacting with our sponsors worldwide.
We are dedicated to ensuring the security of all MMARCRO property and personnel and promoting a safe working environment for employees.
We believe in fair compensation for our employees, and seek fair payment for our services when securing contracts. We do not give or accept gifts or bribes, and firmly believe that hard work should be rewarded in kind.
We meet and exceed all local, national and international laws and regulations regarding clinical trial activities and practices in the regions where we operate, without exception.
We provide all employees with the necessary support to effectively address any ethical concerns which may arise during the conduct of their monitoring activities.Management Policies